Could it be KC (KERATOCONUS)?
KC File #3: KC Masquerading as Myopia
Gloria “Gadget” Chiu, OD, FAAO, FSLS, Los Angeles, CA

 A 33-year-old Asian Indian woman was referred to me for an evaluation. She had a history of soft contact lens wear and although she had always corrected to 20/20 or better, noted that her vision had not been “crisp” for many years. By the time we saw her, she was very unhappy with her vision, particularly in the left eye, complaining of glare and “shadows.” She refracted to 20/20 OD and 20/20- OS, with normal to borderline keratometry readings and clear corneas.
A 33-year-old Asian Indian woman was referred to me for an evaluation. She had a history of soft contact lens wear and although she had always corrected to 20/20 or better, noted that her vision had not been “crisp” for many years. By the time we saw her, she was very unhappy with her vision, particularly in the left eye, complaining of glare and “shadows.” She refracted to 20/20 OD and 20/20- OS, with normal to borderline keratometry readings and clear corneas.
Her contact lens history showed frequent small changes in the prescription and progression of myopia and astigmatism between ages 20-31. During that time, the contact lens prescription for the right eye changed from -1.25 sphere to -3.50 -0.75 x 020 and, for the left eye, from -1.00 sphere to -2.75 -1.25 x 140. Given that myopia typically stabilizes by about age 15,1,2 the degree of myopic progression in this patient’s 20s should have been a clue that something was not right.
The patient’s medical history included asthma, eczema, and seasonal allergies, for which she was treated with an inhaler, topical creams, anti-allergy shots, and eye drops. Keratoconus is associated with all three of these atopic conditions,3 although it is not entirely clear whether atopy and keratoconus share common causative factors or whether corneal ectasia is provoked by eye rubbing due to itching associated with allergies.
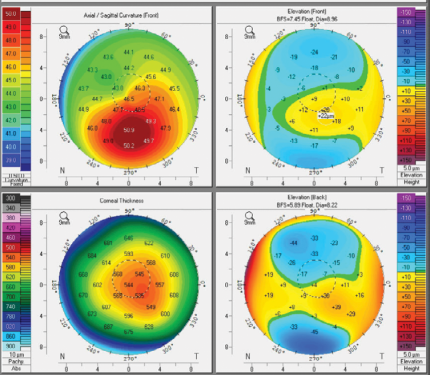
Corneal topography and tomography was performed in this patient for the first time at age 33, during her first pregnancy. This corneal imaging ultimately confirmed the diagnosis of progressive keratoconus; the left eye (Fig 2) was determined to be worse than the right and progressing. Unfortunately, cross-linking of the left eye had to be delayed due to the patient’s pregnancy. Hormonal changes during pregnancy can reduce corneal stiffness and cause or exacerbate an ectatic response.4 iLink cross-linking is contraindicated during pregnancy because of the unpredictability of corneal changes and unknown effect on the fetus of topical drugs used during and after cross-linking.
Following unsuccessful fits with toric soft and hybrid lenses, a scleral lens was able to eliminate the shadows and higher order aberrations she was experiencing in the left eye. After delivery, the patient underwent FDA-approved iLink® cross-linking in her left eye. Both eyes have now been stable for about 7 years, and she wears toric soft contact lenses OU comfortably. She has been prescribed antihistamine eye drops and counseled to not rub her eyes. We continue to monitor her and have begun monitoring her now 7-year old son for signs of KC.
This case illustrates that KC can present with 20/20 vision, low myopia and mild astigmatism, and no obvious changes at the slit lamp. Complaints of “shadows” and vision that is not crisp were key clues, especially in an atopic patient with progressing myopia. The delay in treatment due to pregnancy was unfortunate and could have been avoided with earlier diagnosis.
By following the KC clues that are hiding in plain sight, you can help patients get diagnosed and treated earlier, taking one more concern off your patients’ plate as they become parents themselves. Visit iDetectives.com to learn more.
REFERENCES: 1. PollingJR, et al. BrJ Ophthalmol 2022;106:820-4. 2. The Comet Group. Invest Ophthalmol Vis Sci 2013;54:7871-84. 3. Bawazeer AM, et al. Br J Ophthalmol 2000;84:834-6. 4. JaniD, et al. Clin Exp Optom 2021;104(8):815-25.
Could it be KC (KERATOCONUS)?
KC File #2: Autorefractor Clues That Were Ignored
Susan “Super Sleuth” Gromacki, OD, MS, FAAO, FSLS, First Sight Vision Care

A young woman who had relocated to my area for her first job after college was referred to me by the hometown optometrist who had seen her regularly since childhood. She was a high myope, wearing a spectacle prescription of -4.75 sphere OD and -9.25 -1.25 x003 OS.
The optometrist’s records showed a slow but steady decline in best-corrected visual acuity (BCVA). This went unremarked until 2022, when the patient’s OS could not be corrected even to 20/30. At that point, she was referred to a retina specialist, who ruled out a suspected epiretinal membrane and reported other findings all within normal limits.
In retrospect, there were three clues that could have alerted the practitioner to the possibility of keratoconus. First, when a young person can’t be corrected to 20/20, the cornea is a more likely culprit than the retina. The patient’s vision in the left eye hadn’t been a sharp 20/20 for several years. Secondly, an increase of 0.5 D of astigmatism between annual visits is a significant red flag. And finally, another clue came from the simplest diagnostic tool in the office-the autorefractor. The autorefraction in 2022 showed 2.75 D of astigmatism-a full diopter more than the spectacle prescription-and the quality score was only 8 out of 10. I would expect it to be 10/10 in a healthy young person.
A history of “lazy eye” as a child may be why the declining BCVA in her left eye was not taken very seriously at first. This was noted in her chart, although there was no evidence of binocular vision testing, no mention of exophoria or esophoria, and she didn’t have the typical hyperopic refraction we usually see in a lazy eye. Sometimes practitioners label a worse-seeing eye as a “lazy eye.” While this certainly may be the case, that label threw off her long-time optometrist. The clues could have led the optometrist to a KC diagnosis.
In 2023, when I first saw this patient, corneal topography showed 2.80 D of astigmatism OS with a classic pattern of inferior steepening that is pathognomonic for progressive KC. She was treated with iLink® corneal cross-linking in the left eye and we continue to follow her right eye closely because KC is a bilateral, asymmetric, disease. Recently the patient shared with me that her uncle has KC, something she didn’t know when I asked her initially.
By following the KC clues that are hiding in plain sight, you can help patients like this one avoid years of declining vision-and sleuth out the right specialists to treat the underlying progressive condition that is stealing her sight. Visit iDetectives.com to learn more.
Changing refractive astigmatism and vision:
| YEAR | DC | BCVA |
| 2014 | 1.00 | 20/20 |
| 2015 | 1.25 | 20/20 |
| 2017 | 1.25 | 20/20-1 |
| 2018 | 1.25 | 20/20 |
| 2019 | 1.25 | 20/25 |
| 2021 | 1.25 | 20/20-2 |
| 2022 | 1.75 | 20/30-1 |
| 2023 | 2.25 | 20/30 |
Could it be KC (KERATOCONUS)?
KC File #1: The Patient
Who Corrects to 20/20
Mitch “Private Eye” Ibach OD, FAAO, Vance Thompson Vision

A 29-year-old patient came to our office for a LASIK consult because she was unhappy with fluctuating vision in her contact lenses. The patient had ocular allergies but had no other ocular diagnoses.
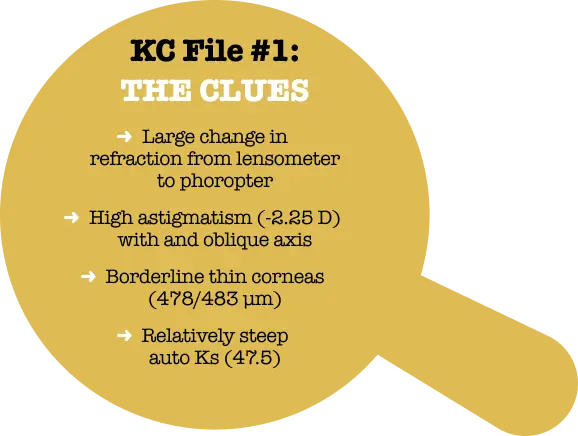
Her entering glasses prescription was a modest one and we were able to refract her to 20/20. However, the refraction in the right eye was our first clue that something was not quite right.
Not only is >2.00 D of refractive cylinder a warning signal for keratoconus, but the oblique axis is also unusual. About 90% of young corneas have with-the-rule (WTR) astigmatism.1 The change in myopic spherical equivalent (SE) from baseline (the glasses prescription) was not what we would expect to see in an adult patient, either.
Refraction and exam findings
| Right Eye | BCVA | Left Eye | BCVA | |
|---|---|---|---|---|
| Lensometry | -0.50 -1.50 x31 | 20/30 | -1.50 -0.50 x172 | 20/20- |
| Refraction at Phoropter | -0.75 -2.25 x34 | 20/20 | -1.75 -0.75 x160 | 20/20+ |
| Pachymetry | 478 μm | 483 μm | ||
| Autokeratometry | 45.5 / 47.50 x 112 | 44.9 / 46.75 x 80 |
REFERENCES
- Kojima T, et al. Am J Ophthalmol 2020;215:127-34,
- Wang Y, et al. Am J Med Genet 2000;93(5):403-9.
- Ferdi AC, et al. Ophthalmology 2019;126(7):935-45.