Lead the investigation and refer patients for iLink® corneal cross-linking*
As an iDetective, be the first on the case to detect progressive keratoconus. Even subtle clues merit a prompt referral for further diagnostics and an iLink® cross-linking consultation to slow or halt the progression of this sight-threatening disease. Following treatment with iLink®, iDetectives continue to maintain their patients’ vision with regular checkups and glasses or contact lenses.
70% of keratoconus
presents through optometry, making you the first line of defense.1
The real risk of keratoconus to patients’ vision
Left untreated, up to
1 in 5 patients
may require a
corneal transplant2
Of those patients,
greater than half
may require multiple transplants within 20 years3
Blow the whistle on keratoconus
Keratoconus could be hiding in plain sight. Be on the lookout for covert clues in the patient history and examination findings that could lead you to suspect keratoconus, and be ready to sound the alarm. It’s time to #FollowTheClues.
Mission Possible: Keep your patients on the right track
In the keratoconus journey, it’s up to you to ensure that patients you suspect have keratoconus get the optimized care they deserve.
Collect subtle clues during the comprehensive eye exam.
Refer KC suspects for a cross-linking consultation.
Solve their vision needs.
Discover what to expect during post–cross-linking management
Help solve the case:
When in doubt, refer out
iDetectives can spot the clues and promptly refer patients with suspected progressive keratoconus to a corneal specialist who can confirm whether treatment with iLink® is appropriate.
- Delaying treatment of progressive keratoconus may result in continued progression and loss of vision4
- Early intervention may reduce strain on patients’ financial resources and increase their quality of life
- iLink® offers patients peace of mind knowing their vision can be preserved by slowing or halting disease progression with an FDA-approved device
Both the American Academy of Ophthalmology and the Cornea Society recommend cross-linking for the treatment of progressive keratoconus.
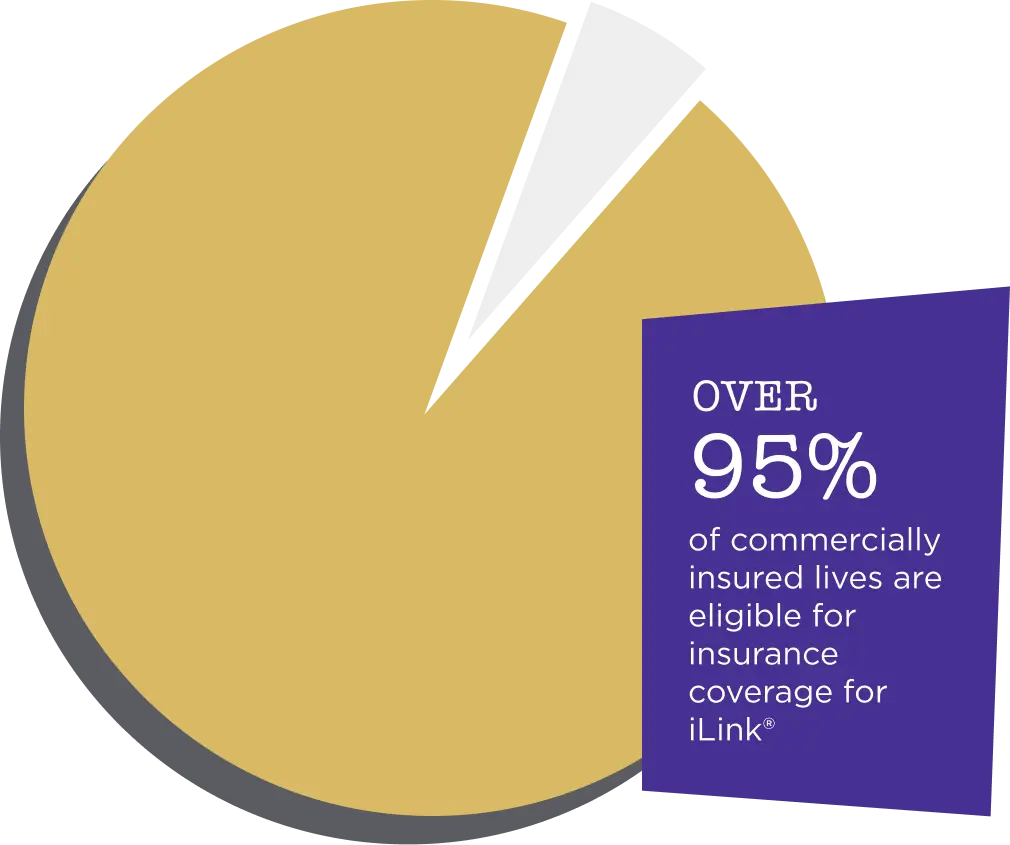
Stop keratoconus in its tracks
Only iLink®:
- Is an FDA-approved corneal cross-linking procedure
- Is widely covered by insurance
- Slows or halts progressive keratoconus with proven technology, efficacy,
and safety - Is backed by 20 years of science to achieve the right formulation
94% of iLink® corneal cross-linking cases have been shown to slow or halt the progression of keratoconus.5
Make a referral you can trust—your patients will thank you for it
If it’s not iLink®, it’s not approved by the FDA. Find a specialist who offers FDA-approved iLink®. Watch out for false flags! Don’t be double-crossed by unapproved cross-linking drugs and devices.
After you refer a patient, stay on the case to solve their vision needs. Early intervention with iLink® helps unlock the refractive toolbox and may be able to prevent patients from becoming intolerant to contact lenses.
ENSURE THEY UNDERSTAND THAT:
- iLink® is covered by the majority of commercial insurance providers
- iLink® is a minimally invasive outpatient procedure
- Patients are typically awake during the 1-hour procedure
- Some discomfort is possible during immediate recovery
- iLink® is not intended to improve their vision
- You will monitor their recovery and vision needs after treatment
*Using Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution), Photrexa® (riboflavin 5’-phosphate ophthalmic solution), and the KXL® system, the iLink® corneal cross-linking procedure from Glaukos is the only FDA-approved therapeutic treatment for patients with progressive keratoconus and corneal ectasia following refractive surgery.†6
†Photrexa® Viscous and Photrexa® are manufactured for Avedro. The KXL® system is manufactured by Avedro. Avedro is a wholly owned subsidiary of Glaukos Corporation.
REFERENCES
- Eisenberg JS. First treatment for keratoconus itself. Optometry Times. June 1, 2012. Accessed April 25, 2023.
https://www.optometrytimes.com/view/first-treatment-keratoconus-itself. - Pramanik S, Musch DC, Sutphin JE, Farjo AA. Extended long-term outcomes of penetrating keratoplasty for keratoconus. Ophthalmology. 2006;113(9):1633-1638.
- Maharana PK, Agarwal K, Jhanji V, Vajpayee RB. Deep anterior lamellar keratoplasty for keratoconus: a review. Eye Contact Lens. 2014;40(6):382-389.
- Romano V, Vinciguerra R, Arbabi EM, et al. Progression of keratoconus in patients while awaiting corneal cross-linking: a prospective clinical study.
J Refract Surg. 2018;34(3):177-180. - Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK; United States Crosslinking Study Group. United States Multicenter Clinical Trial of Corneal Collagen Crosslinking for Keratoconus Treatment. Ophthalmology. 2017;124(9):1259-1270.
- Photrexa. Prescribing information. Avedro; 2022.